Which of the Following Best Describes a Single Replacement Reaction
Which of the following best represents the reaction between sulfuric acid and calcium hydroxide. See answers 2 Best Answer.

Single Replacement Reaction Definition And Examples
Balance polyatomic ions as a single unit check each reactant and product to verify the coefficients.
. Add your answer and earn points. Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas. ZnCuCl2 Cu ZnCl2.
All of the above. A halogen replaces another halogen that is in solution. Because Magnesium Replaces Hydrogen.
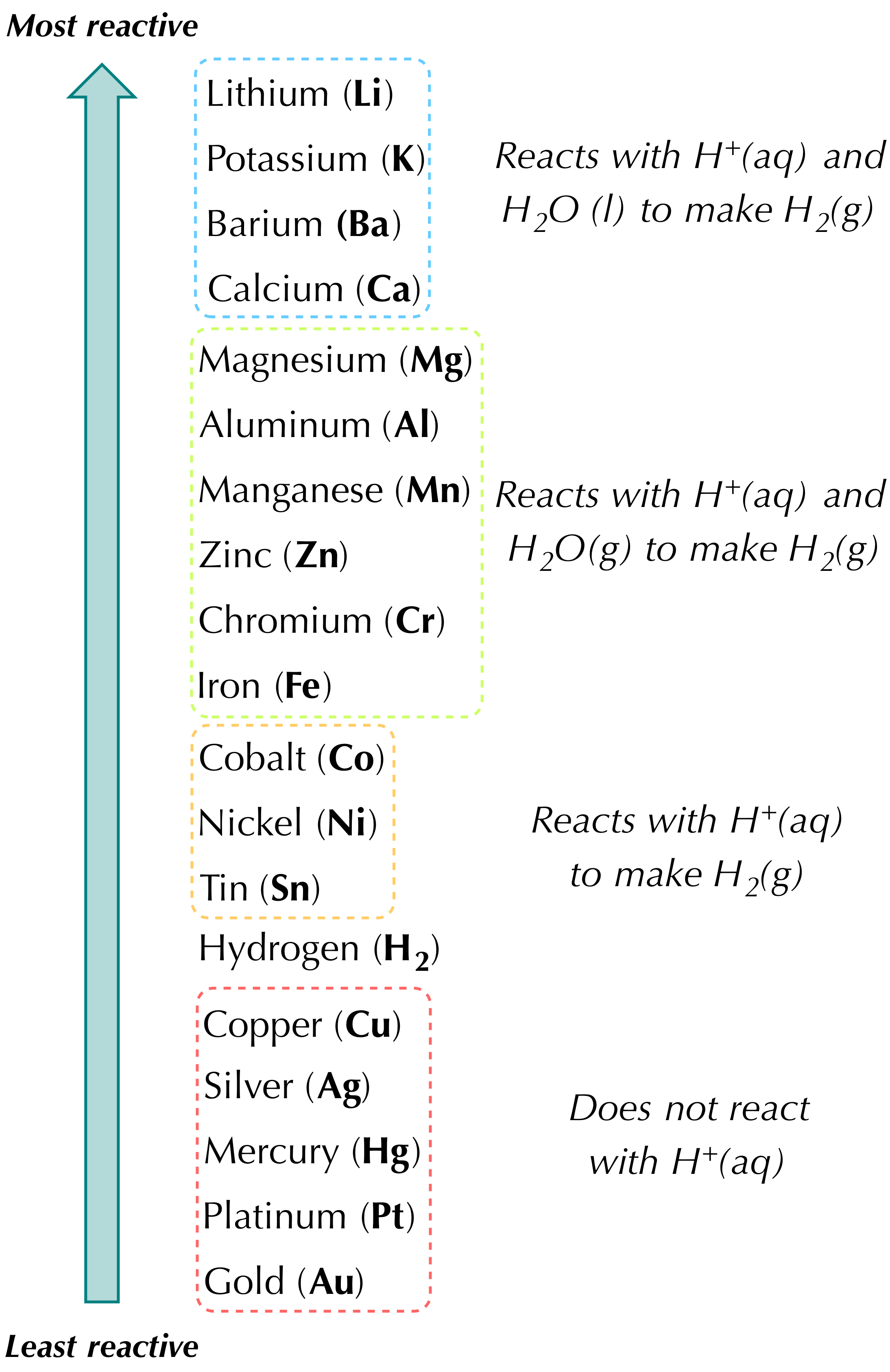
Two elements combine to form a compound. Combination reaction C single-replacement reaction D double-replacement reaction E neutralization reaction. A metal replaces another metal that is in solution.
Mg 2HCl MgCl2 H2. ABC B AC. A compound breaks apart into separate elements.
The three general categories of single-replacement reactions are. 2 on a question. Which reaction type best describes the.
Fes CuSO. One element takes the place of another in a compound B. In other words it is where an element reacts with a compound and replaces one component of the compound.
What are the products from the following single-replacement reaction. Hydrogen usually forms the cation in a single replacement reaction. Which of the following is a single replacement reaction.
CaC 2 s H 2 Ol HCCHg CaOs Ans. What type of chemical reaction is illustrated in the following example. What will happen to the height h of the.
Simpler compounds from a complex compound. A single-replacement reaction also called a single-displacement reaction is one in which a pure element and a compound react chemically so that the products include another pure element and a different compound via exchanging of two species. Zns CuSO4aq - Cu and ZnSO4.
Two elements switch places in. The potential energy of the reactants is greater than that of the surroundings. Which of the following best describes a single replacement reaction.
HClaq KOHaq KClaq H2Ol. Predict the products of the following single replacement reaction. Describe the procedure used to make 30 liters of a 20 M KCl solution starting with solid KCl and water.
The following reaction is a redox reaction. Which of the statements below best describes the following reaction. Which of the statements below best describes the following reaction.
The reaction above is classified as. Which of the following best describes a single-replacement reaction. MathrmZns2 mathrmHCla q rightarrow mathrmZnCl_2a qmathrmH_2g The reaction can be made to occur more slowly by 1 raising the temperature and using a single piece of zinc rather than powdered zinc of the same mass 2 lowering the temperature and using a single piece of zinc rather.
A compound breaks apart into separate elements C. Two elements combine to form a compound D. Note the arrow has this symbol Δ over it Δ Na2CO3s Na2Os CO2g Sodium carbonate is heated to give sodium oxide and carbon dioxide.
One element takes the place of another in a compound. ABC C BA. Heat must be added to get the reaction to go B.
There are two types of single replacement reactions. 1 See answer Advertisement Advertisement laurenkropp is waiting for your help. Question 3 of 10 Which of the following best describes a single-replacement reaction.
F H2 I2 2HI G 2NaCl 2Na Cl2 H NaF HCl HF NaCl. Which of these reactions shows simple chemical decomposition. The temperature of the surrounding decreases C.
Which of the following best describes an exothermic reaction. The common non-metals in single replacement reactions are the group 17 elements which generally form anions with a 1- charge. Two elements switch places in two compounds.
Well its D Mg 2HCl ----- MgCl2 H2. Matter is converted into energy E. Which of the statements below best describes the following reaction.
Which of the following describes a single-replacement reaction. Sodium carbonate decomposes with heat. C Single replacement D Combustion.
One element takes the place of another in a compound. Two elements combine to form a compound one element takes the place of another in a compound two elements switch places in a compound a compound breaks into separate elements. F H2SO4 CaOH2 CaSO4 H2O.
Heat is absorbed by the reaction from the surroundings. Consider the following equation.

Single Replacement Reactions Article Khan Academy

Combustion Reactions Chemical Reactions Chemical Equation Reactions

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube
No comments for "Which of the Following Best Describes a Single Replacement Reaction"
Post a Comment